A proteins structure determines how well it functions. Protein Structure Part 2.

Proteins must fold to their active native state when they emerge from the ribosome and when they repeatedly unfold and refold during their lifetime 1 2.
Why do proteins fold. What are proteins and why do they fold. What is the physical code by which an amino acid sequence dictates a proteins native. The promise of AI.
Perhaps its the scale that AI brings to the field the ability to do. Some proteins bond together to lend rigidity to the cell and shape neurons muscles organs and more. Other proteins act as catalysts for chemical reactions or serve as transportation for other molecules.
Whatever their function all proteins exhibit folding which enables each protein to perform its job within the cell. What Is Protein Folding. It is believed that proteins fold in order for it to achieve the lowest potential energy it needed to arrive at its targeted shape.
Proteins can fold into. 1 Proteins do fold yes but that folding is dependent on the environment. 2 Proteins are extremely dynamic.
They have no one 3D structure. In vivo there are proteins which exist in. 3 There is such a thing as intrinsically unstructured proteins.
4 There are 2 things that. Protein Structure Part 2. How and Why Do Proteins Fold.
If playback doesnt begin shortly try restarting your device. Videos you watch may. Protein folding can go wrong for three major reasons.
A person might possess a mutation that changes an amino acid in the protein chain making it difficult for a. On the other hand protein folding failure can be viewed as an ongoing and. What are proteins and why do they fold.
The Google company DeepMind says its artificial intelligence system AlphaFold can predict the structure of proteins. A proteins structure determines how well it functions. Heres why thats important for your health.
2020-12-08 - The Google company DeepMind says its artificial intelligence system AlphaFold can predict the structure of proteins. Proteins are linear sequences of amino acids. But with very few exceptions in order to implement their biological function they need to fold into a well-defined 3-dimensional structure.
The ability to do this and the resulting structure depends crucially on not just the overall amino acid content of the polypeptide but its specific sequence. In effect the information required to enable a polypeptide to fold into a protein. Why Proteins Fold Proteins are the action superheroes of the body.
As enzymes they make reactions go a million times faster. As versatile transport vehicles they carry oxygen and antibodies to fight disease. They do a thousand different jobs and with no complaint.
But before a protein can go to work it must fold into the right shape. Proteins are folded and held together by several forms of molecular interactions. The molecular interactions include the thermodynamic stability of the complex the hydrophobic interactions and the disulfide bonds formed in the proteins.
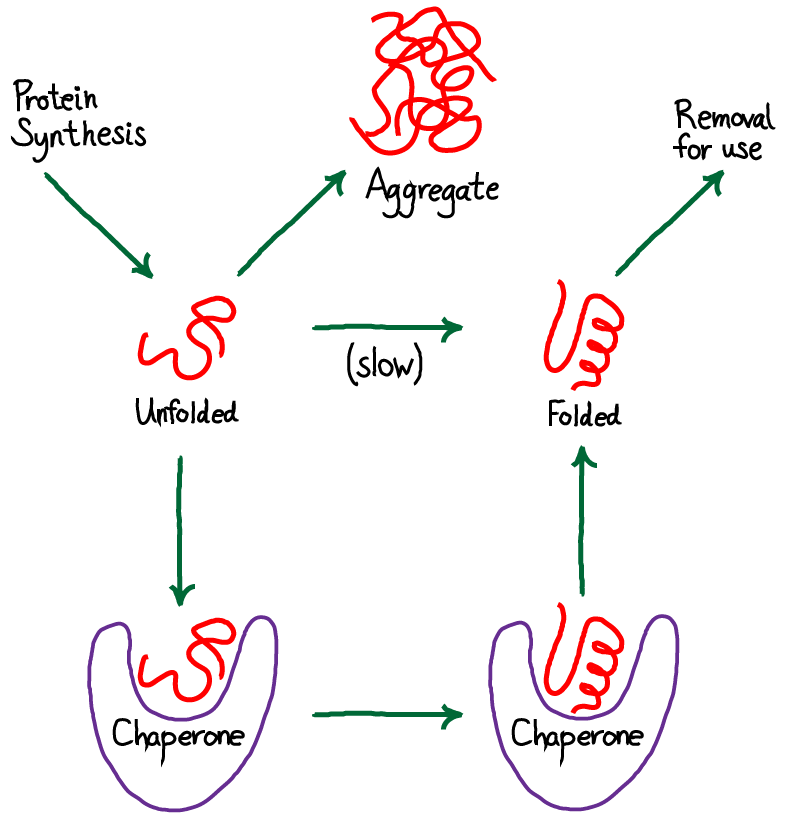
The figure below Figure 2 is an example of protein folding. Proteins must fold to their active native state when they emerge from the ribosome and when they repeatedly unfold and refold during their lifetime 1 2. The folding process is difficult 3 4 and potentially dangerous 5.
Biological health depends on its success and disease on its failure. On this basis Anfinsen framed his famous Thermodynamic hypothesis that protein folding just like any other spontaneous chemical process simply proceeds energetically downhill to its lowest free energy form the native state. A protein is a string of amino acids and what it does is determined by the shape it takes.
That shape is determined by the sequence of the amino acids. Like a piece of biological origami the protein folds itself into the form necessary to carry out its job. Without the shape the protein would be worthless.
We have shown that water is the key to proteins ability to quickly find their native fold. Within each protein there are regions of the protein chain that can form favorable interactions with water. These regions tend to remain on the exterior of the folded protein in order to make contact with water.