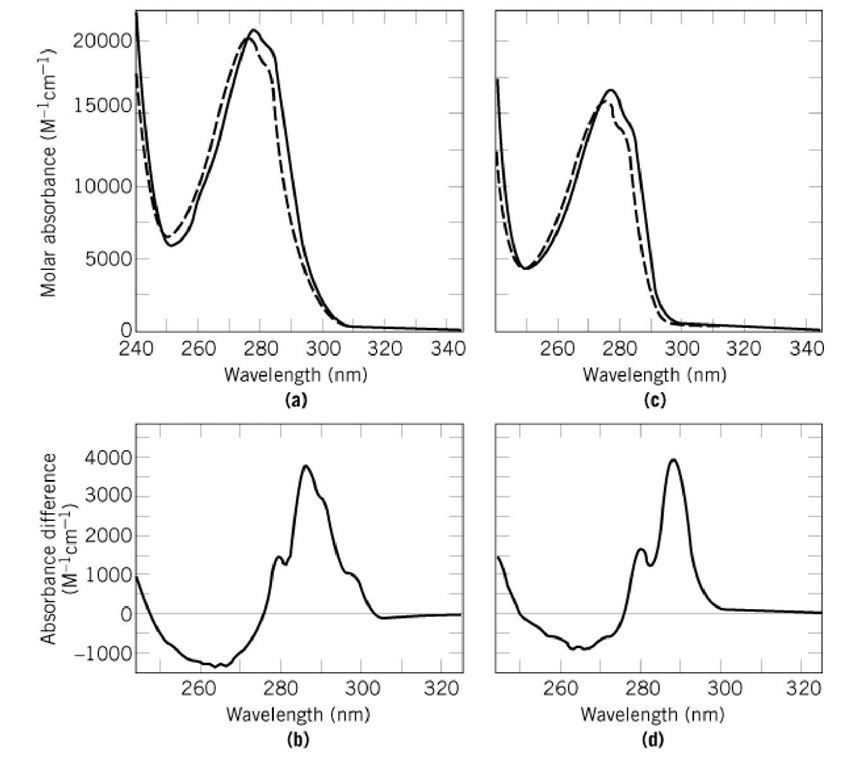
Fraction transmitted. Absorbance at 280 nm by aromatic side chains is frequently used to monitor conformational changes in proteins as well as to determine protein concentrations.
Our previous studies suggest that proteins rich in charged amino acids may also absorb between 250 and 350 nm even if they lack aromatic amino acids.
Uv absorbance of amino acids. UV absorption of Amino Acids. What is absorbance of a 001 M solution of phenylalanine if path is 1 cm. A εcx A 100 x 01 x 1 1.
Fraction transmitted. What is absorbance of a 001 M solution of tyrosine is path if 1 cm. A 1000 x 01 x 1 10.
Fraction transmitted. This absorption is due to the aromatic amino-acids present in the protein. The advent of quantitative methods of spectrophotometry is the basis of a method of determining tyrosine and tryptophan in proteins.
The striking property of proteins is their transparency indicating a high degree of electronic saturation. The configurational stability of the protein molecule depends entirely on extra-valence. There are two amino acids that are primarily responsible for the UV absorbance of proteins.
Both have a molar absorption coefficient at a wavelength of 280 nm but the peak absorbance of the protein itself is largely determined by the arrangement and concentration of these specific amino acids within the linear chain. Proteins absorb strongly at 280 nm due to three types of its constituent amino acids. The peptide bonds found in the amino acids also absorb at 205 nm.
The UV absorption of protein can be used both to quickly image and acquire spectra of microscopic samples non-destructively. The spectra can also be used to determine protein concentrations and the relative amounts of protein to DNA or RNA. In addition the indirect UV detection at 264 nm was achieved in a BGE of 20 mmolL Na2HPO4 100 mmolL p-aminosalicylic acid PAS as UV absorbing probe and 400 mmolL β-CD at pH 122.
The β-CD significantly benefited the isomeric separation of Leu L- and D-Ile. The optimal conditions allowed the LODs limit of detections of direct and indirect UV absorption detection to be. In the near UV 290 nm all the tested acids show a common absorption maximum and good sensitivities.
In the visible region at 404 nm the sensitivities of the α-amino acids are very similar to those observed at 570 nm. At 520 nm the αamino acids have the lowest sensitivities. The amino acid dithiocarbamates formed are water soluble and show UV absorption at ca.
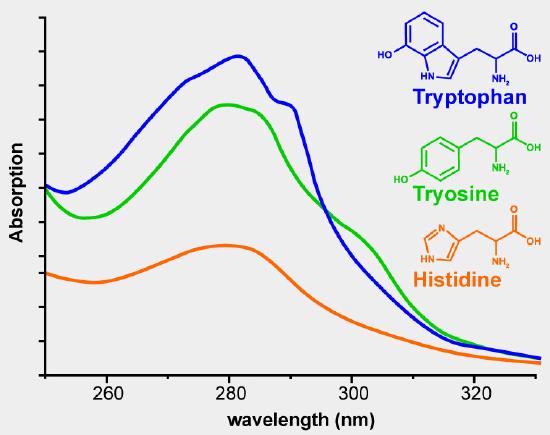
255 and 285 rim EXPERIMENTAL Apparatus and reagents UV measurements were made in a 1-cm quartz cell in a Hitachi UV 150 spectrophotometer with a UV 150-20 data processor. Deionized water was used throughout. Proteins usually show absorption maxima between 275 and280nmFigure 1whicharecausedbytheabsorbance of the two aromatic amino acids tryptophan Trp and tyrosineTyrandtoasmallextentbytheabsorbanceof cystineieofdisulfidebondsTheabsorbancesofTrp and Tyr depend on the microenvironment of their chromophores and they are slightly red-shifted when.
As demonstrated in Figure 2 aromatic amino acids and proteins absorb UV light with two distinct peaks. The peak centered on 280 nm is the result of absorbance by the aromatic ring portion of their structure. The peak at lower wavelengths is caused by absorbance of peptide and carboxylic acid moieties in the compounds.
On a molar basis tryptophan absorbs more light at. The relationship between protein concentration and UV absorbance is complicated by a number of factors. Different amino acids absorb at different wavelengths The extinction coefficients differ widely The amino acid composition of proteins varies widely Nucleic acids absorb strongly near 260 nm.
Studies suggest that proteins rich in charged amino acids may also absorb between 250 and 350 nm even if they lack aromatic amino acids10 Establishing a quantitative link between non-aromatic protein amino acid sequence content and the UV-Vis absorption. Our previous studies suggest that proteins rich in charged amino acids may also absorb between 250 and 350 nm even if they lack aromatic amino acids. 10 Establishing a quantitative link between non-aromatic protein amino acid sequence content and the UV-Vis absorption features above 320 nm would open up a new spectral window to probe prominent proteins of biomedical relevance.
Abstract and Figures In current study we report efficient and clean procedure for preparing mycosporine-like amino acids MMAs analogs and evaluate their ultraviolet absorbance properties and. Complementary UV-absorption of mycosporine-like amino acids and scytonemin is responsible for the UV-insensitivity of photosynthesis in Nostoc flagelliforme Mycosporine-like amino acids MAAs and scytonemin are UV-screening compounds that have presumably appeared early in the history of life and are widespread in cyanobacteria. Cence UV absorption is also known to be sensitive to the conforma-tional changes in proteins 34.
Absorbance at 280 nm by aromatic side chains is frequently used to monitor conformational changes in proteins as well as to determine protein concentrations. Absorbance at 230 nm A 230 is also known to be sensitive to protein conformation 49. UV spectra of protein solutions usually.