Syngaschem BV is a small research enterprise established in 2013 in NuenenEindhoven The Netherlands. In Figure 1 the words synthesis gas have been shown as the source of two products ammonia and methanol.
But ammonia requires nitrogen which is obtained from the producer gas by causing it to undergo the water-gas.
Synthesis gas chemical formula. In Figure 1 the words synthesis gas have been shown as the source of two products ammonia and methanol. It is not quite the same synthesis gas in the two cases but they are closely related. The mixture of carbon monoxide and hydrogen.
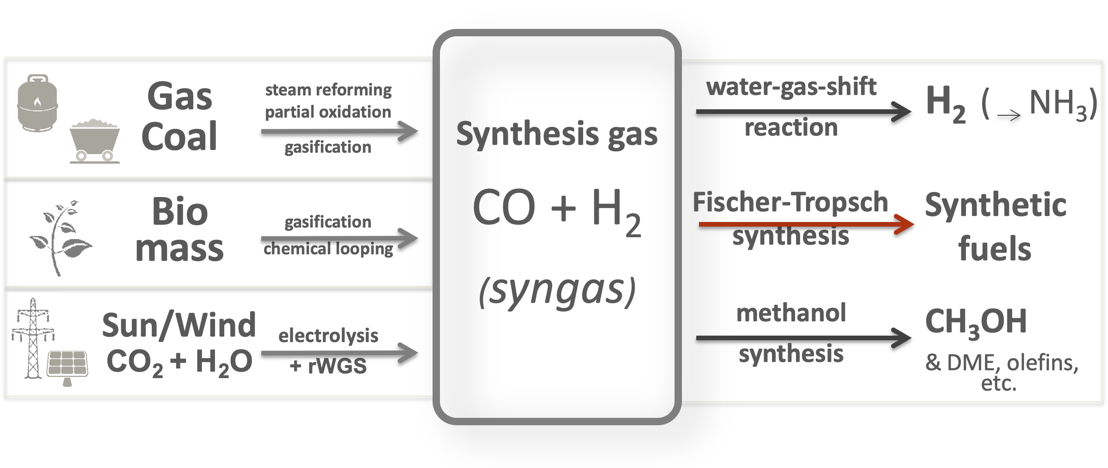
Synthesis gas also known as syngas is mainly composed of CO H 2 and CO 2. In addition it may contain other gases such as nitrogen and methane. It is produced through a thermochemical process called gasification which converts carbonaceous materials such as biomass municipal wastes coal petroleum and tires under controlled amount of oxidant such as oxygen air and CO.
The general chemical equation for a synthesis reaction is A B AB The reaction of a metal with a non-metal to produce a compound is an example of a synthesis reaction. Examples of synthesis reactions 1. Synthesis gas is a very important chemical intermediate for many relevant processes including the production of methanol and Fischer-Tropsch synthesis of synthetic fuels.
Syngas is currently produced by the endothermic steam reforming of methane or by homogeneous oxidation in autothermal reforming. There has recently been significant interest in alternative routes to syngas production and one of the. Unlike FischerTropsch synthesis Chapter 10 Section 43 CO is not polymerized.
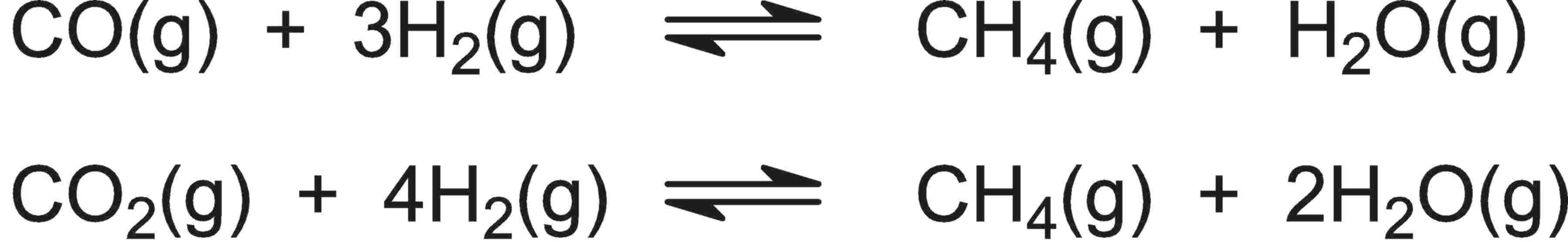
109 is a very exothermic reaction and the conversion is equilibrium limited. The equilibrium constant for methanol synthesis can be calculated by Eq. 109CO 2 H2 CH3OH ΔH 91 kJ mol1.
Synthesis and reactions Mustard gas is the organic compound with formula ClCH 2 CH 2 2 S. In the Depretz method mustard gas is synthesized by treating sulfur dichloride with ethylene. SCl 2 2 C 2 H 4 ClCH 2 CH 2 2 S.
One way of dealing with the excess hydrogen is to inject carbon dioxide into the methanol synthesis reactor where it too reacts to form methanol according to the equation. CO 2 3 H 2 CH 3 OH H 2 O displaystyle ce CO2 3 H2 - CH3OH H2O. Phosgene is the organic chemical compound with the formula COCl 2It is a colorless gas.
In low concentrations its odor resembles that of freshly cut hay or grass. Phosgene is a valued industrial building block especially for the production of precursors of polyurethanes and polycarbonate plastics. Phosgene is very poisonous and was used as a chemical weapon during World War I where it was.
Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a slight. This is a list of common chemical compounds with chemical formulae and CAS numbers indexed by formulaThis complements alternative listing at list of inorganic compoundsThere is no complete list of chemical compounds since by nature the list would be infinite.
The figure of gasification reactions and transformations illustrated the concept of coal gasification and noted resulting composition of syngas. This can vary significantly depending on the feedstock and the gasification process involved. However typically syngas is 30 to 60 carbon monoxide CO 25 to 30 hydrogen H 2 0 to 5 methane CH 4.
Chemical industry - Chemical industry - Synthesis gas. In Figure 1 the words synthesis gas have been shown as the source of two products ammonia and methanol. It is not quite the same synthesis gas in the two cases but they are closely related.
The mixture of carbon monoxide and hydrogen described above is the synthesis gas that is the source of methanol. But ammonia requires nitrogen which is obtained from the producer gas by causing it to undergo the water-gas. Methanethiol ˈmɛθeɪnˈθaɪɒl is an organosulfur compound with the chemical formula CH 3SH.
It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood brain and feces of animals as well as in plant tissues. It also occurs naturally in certain foods such as some nuts and cheese.
It is one of the chemical compounds responsible for bad breath and the smell of flatus. Syngaschem BV is a small research enterprise established in 2013 in NuenenEindhoven The Netherlands. Its mission is to promote the utilization of synthesis gas on the basis of molecular scale understanding.
Syngaschem BV engages in fundamental research on the catalysis and surface science of syngas conversion with focus on the responsible use of. Styrene C6H5CHCH2 or C8H8 CID 7501 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Search chemicals by name molecular formula structure and other identifiers.
Find chemical and physical properties biological activities safety and toxicity information patents literature citations and more. We are constantly adding new data and working on improving interfaces to chemical information. Please check back often.
Of the three methanol synthesis reactions the latter is the well-known water-gas-shift WGS reaction. Since the H 2 CO ratio in syngas from todays slagging gasifiers typically ranges from 03 to 1 extensive water gas shift is required to meet the stoichiometric H 2 CO ratio of 2 for full conversion to methanol.