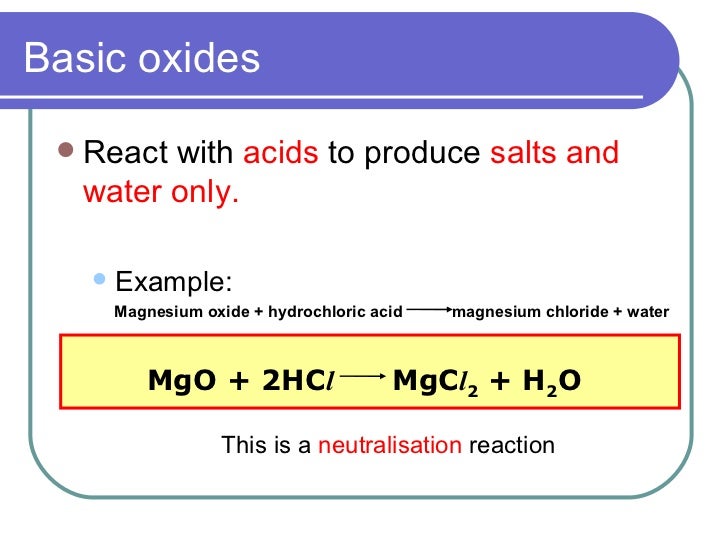
These compounds form phosphates which stabilize the pH of. 1 iiExplain why an excess of magnesium oxide can be used for this neutralisation.
The mixture produced was then disposed of in a lake.
Magnesium oxide and phosphoric acid. Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. Magnesium hydroxide react with phosphoric acid. If the reaction has an expected yield of 81 how much magnesium oxide should be reacted to produce 980 g of magnesium hydroxide.
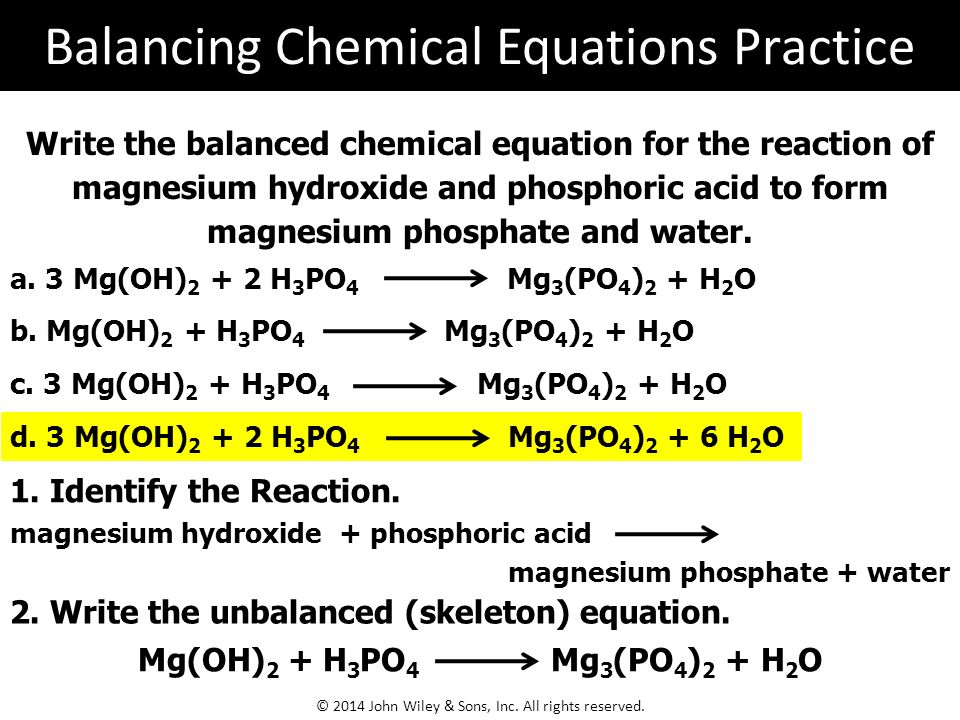
Magnesium Oxide Phosphoric Acid Magnesium Phosphate Water Bi2O3 ClO- OH- BiO3- Cl- H2O. Phosphoric acid reacts with magnesium carbonate to produce. Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the following reactionH3PO4 MgOH2-Mg3PO42 H2O.
Magnesium react with phosphoric acid 3Mg 2H 3 PO 4 Mg 3 PO 4 2 3H 2 Check the balance Magnesium react with phosphoric acid to. Chemical reactions Сhemical tables. Firstly write out the BALANCED equation or youre getting nowhere.
ChlorineI oxide is far less. The phosphate bonds in contrast to orthophosphoric acid reduce the quantity of magnesia-phosphate glass in the specimens by a factor of 153. The aluminophosphate bond to a greater degree contributes to recrystallization of the periclase compared with H3PO4 magnesium- and chromium-phosphate bonds.
Phosphoric acid or phosphates ionize in solution to form H2PO4 HPO42 andor PO43 and these anions adsorb onto Mg OH H2Ox to inhibit the formation of Mg OH2 and further promote the. The resulting phosphoricV acid solution was neutralised using an excess of magnesium oxide. The mixture produced was then disposed of in a lake.
I Write an equation for the reaction between phosphoricV acid and magnesium oxide. 1 iiExplain why an excess of magnesium oxide can be used for this neutralisation. Reaction with acids.
Magnesium oxide reacts with acids as you would expect any simple metal oxide to react. For example it reacts with warm dilute hydrochloric acid to give magnesium chloride solution. Describing the properties of aluminium oxide can be confusing because it exists in a number of different forms.
Magnesium oxide is another simple basic oxide which also contains oxide ions. However it is not as strongly basic as sodium oxide because the oxide ions are not as weakly-bound. In the sodium oxide the solid is held together by attractions between 1 and 2- ions.
In magnesium oxide the attractions are between 2 and 2- ions. Magnesium oxide reacts with phosphoric H3PO4 TO PRODUCE MAGNESIUM PHOSPHATE AND WATER. HOW MANY GRAMS OF MAGNESIUM OXIDE ARE REQUIRED TO REACT COMPLETELY WITH 335G OF PHOSPORIC ACID.
A 45 g piece of magnesium ribbon undergoes combustion in air to produce a mixture of two ionic solids MgO and Mg3N2. Water is added to this. Dimethyl Thio toluene Diamine.
Our enhancement depends around the sophisticated devices exceptional talents and repeatedly strengthened technology forces for Magnesium Oxide And Phosphoric Acid Ethyl Silicate Uses Isodecyl Diphenyl Phosphate Dmtda And we can help looking for any products of the customers needs. 2 Mg s H2SO4 aq Mg2SO4 aq H2 g note the key here is DILUTE sulphuric acid to yield the weak redox products. 3Mgs 2H3PO4aq — 3H2g Mg3PO42sI believe the equation is.
Mg H3PO4 — H2 Mg3PO4. How much does does a 100 dollar roblox gift card get you in robhx. For example C6H5C2H5 O2 C6H5OH CO2 H2O will not be balanced but XC2H5.
Small quantities of other oxides such as magnesium oxide may also be present. The liquid is essentially an aqueous solution of phosphoric acid buffered by adding small quantities of zinc oxide or aluminium oxide. These compounds form phosphates which stabilize the pH of.
Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the following reactionH3PO4 MgOH2 —– Mg3PO42 H2OBalance the. Phosphoric acid with magnesium oxide and drying of the resultant product. Chemical names Monomagnesium dihydrogen phosphate CAS.
Number 13092-66-5 Anhydrous 15609-87-7 Dihydrate Chemical formula Mg H 2PO 4 2. X H 2O x 0 to 4 Formula. Magnesium Hydroxide for Removal of Ammonia and Phosphorus in Wastewater.
Magnesium hydroxide is soluble in dilute acid and ammonium salt solution. It is almost insoluble in water and alcohol. Solubility 18 is 00009 g.
Magnesium and aluminum impurities may be removed from wet process phosphoric acid by adding a fluoride ion donating compound such as hydrofluoric acid for example to thereby provide about four fluoride ions for each aluminum ion. Also preferably an aluminum ion donating compound such as alum is added to the wet process acid to bring the aluminum ion to magnesium ion ratio up to about two.