
A FeS iron-sulfur cluster. This formula means that in ANY AMOUNT of pyrrhotite the ratio of iron to sulfur will be 11.
Iron sulfides are often iron-deficient non-stoichiometric.
Iron and sulfur formula. FeS2 is the chemical formula for iron and sulfur. The name ofthe compound is ironII sulfide because the iron ion has a chargeof 2. Sulfur dioxide SO 2 g TOXIC is formed if the sulfur catches fire see CLEAPSS Hazcard HC097.
Prepare a mixture containing iron powder and sulfur powder in the ratio 74 by mass. Do this by weighing out 7 g of iron powder and 4 g of finely powdered sulfur onto separate pieces of filter paper or use weighing boats. Mix the two powders by pouring repeatedly from one piece of.
It is assumed that the main reaction that occurs is. Fe s S s – FeS s but the system is probably much more complex than that. The standard enthalpy of formation of FeS is -100 kJmol.
001- Iron Sulfur Iron II Sulfide. If playback doesnt begin shortly try restarting your device. Sulfur in British English.
Sulphur is a chemical element with the symbol S and atomic number 16. It is abundant multivalent and nonmetallicUnder normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8Elemental sulfur is a. IronIII sulfide decays at a temperature over 20 C into ironII sulfide FeS and elemental sulfur.
Fe 2 S 3 2 FeS S With hydrochloric acid it decays according to the following reaction equation. Iron-sulfur FeS centers are essential protein cofactors in all forms of life. In particular FeS centers function as enzyme cofactors in catalysis and electron transfer and they function as sensors of environmental conditions.
Moreover they are indispensable for the biosynthesis of other protein cofactors including complex metal-clusters. Prominent examples are radicalS-adenosylmethionine-dependent. Iron sulfide has the ideal formula FeS Fe iron S sulfur.
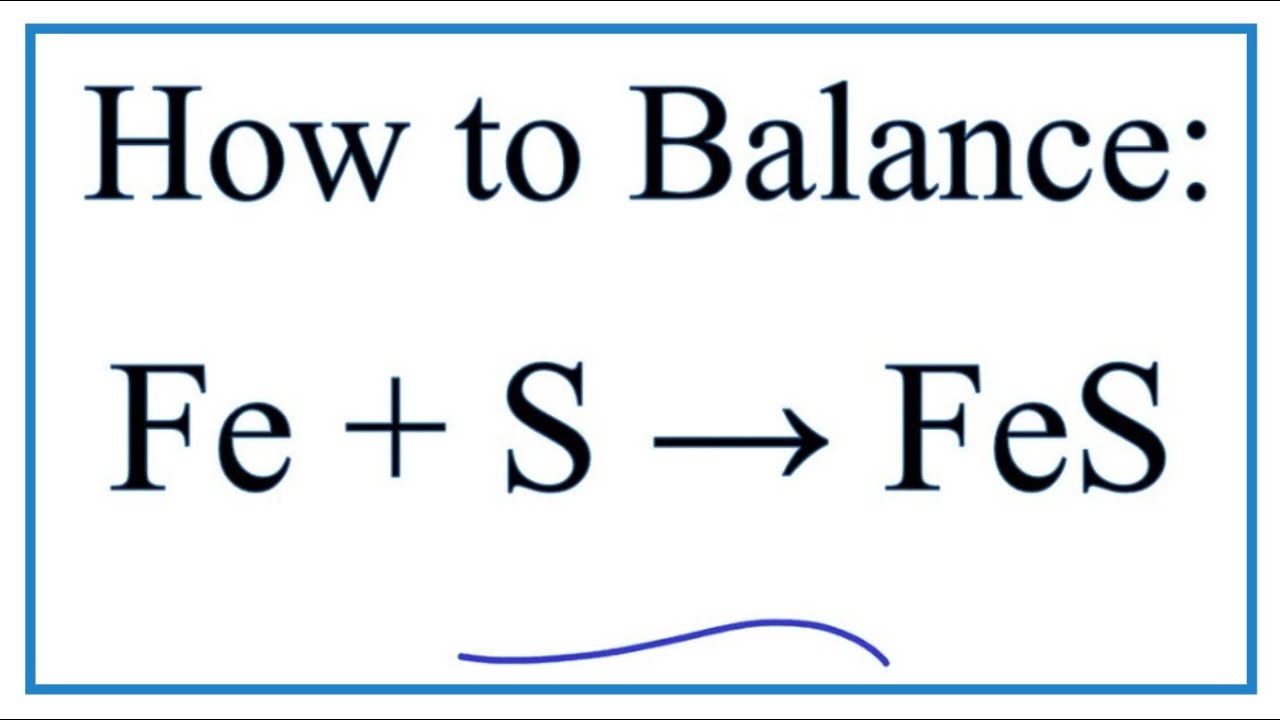
This formula means that in ANY AMOUNT of pyrrhotite the ratio of iron to sulfur will be 11. The equation for the reaction that produces the iron sulfide is. Fe S FeS heat.
It is a compound of sulfate and iron in where the ratio of iron to sulfate ion is 11. Some hydrates that occur naturally are monohydrate and tetrahydrate. The molecular or chemical formula of Iron II sulfate is FeSO 4.
Iron II sulfate is a crystalline solid either greenish or yellow-brown. The molecule iron III sulfide is an ionic molecule between the metal Iron and the non-metal sulfur. In an ionic molecule the formula is determined by balancing the charges on the ions.
For this case the roman numeral III tells us the iron has a charge of 3 or F e3 The the element sulfur has a charge of 2 or S2. EPA Chemicals under the TSCA. European Chemicals Agency ECHA.
Hazardous Substances Data Bank HSDB 232 Related CAS. A FeS iron-sulfur center. A FeS iron-sulfur cluster.
IronII sulfide 99-Fe IronII sulfide fused sticks. IronII sulfide sticks thin IronII sulfide technical grade. IronII sulfide 999-Fe 8658AF.
IronII sulfide VetecTM reagent grade. Iron Carbon Fe-C Alloy Sputtering Targets. IronIII sulfate or ferric sulfate is a family of inorganic compound with the formula Fe 2 SO 4 3 H 2 O n.
A variety of hydrates are known in fact are the most commonly encountered form of ferric sulfate. Solutions are used in dyeing as a mordant and as a coagulant for industrial wastes. It is also used in pickling baths for aluminum and steel.
Generally ferric sulfate. Iron II sulfide or ferrous sulfide BrE. Sulphide is one of a family chemical compound s and mineral s with the approximate formula ironFe sulfideS.
Iron sulfides are often iron-deficient non-stoichiometric. All are black water-insoluble solids. Read full article at Wikipedia.