
Lewis Structure is also known as an electron-dot structure since we use dot notations to represent the valence electrons surrounding the atoms. Count the number of bonding pairs and the number of lone pairs around the hydrogen atom.
Molecular Structure of Hydrogen Iodide.
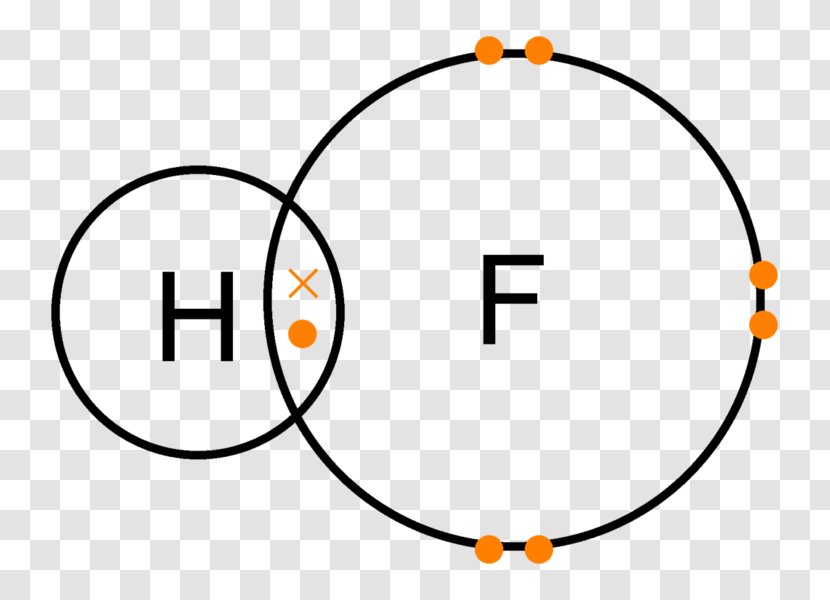
Hydrogen fluoride lewis structure. Hydrogen fluoride is a diatomic molecule so here the concept of a central atom does not take place. We can place them accordingly. Lewis Structure is also known as an electron-dot structure since we use dot notations to represent the valence electrons surrounding the atoms.
Hydrogen and Fluorine are both non-metals so they form a COVALENT bond and SHARE electrons to complete their outer shells. Hydrogen shares one electron with. Bonding Molecular Structure Page 44 Lewis Dot Structure of Hydrogen Fluoride.
Drawing Lewis Structures Sum the valence electrons from all atoms in the species. Write the atomic symbols for the atoms involved so as to show which atoms are connected to which. Draw a single bond between each pair of bonded atoms.
Below is the Lewis structure of the hydrogen fluoride HF molecule. Count the number of bonding pairs and the number of lone pairs around the hydrogen atom. Our mission is to help you succeed in your Chemistry class.
HYDROGEN FLUORIDE HF Molecular Formula H 2 F 2. Average mass 40013 Da. Monoisotopic mass 40012455 Da.
Hydrogen fluoride is a colorless corrosive liquid or gas and is composed of a hydrogen atom and a fluorine atom. It has a strong irritating odor. Hydrogen fluoride readily dissolves in water and is referred to as hydrofluoric acid HFA in its dissolved form.
It is present in a variety of over-the-counter products at concentrations of 612. Although HFA is weak compared with most other mineral acids. Exposure requires immediate medical attention.
COVID-19 is an emerging rapidly evolving situation. Solid HF consists of zig-zag chains of HF molecules. Will form explosive mixtures with air.
Vapors from liquefied gas are initially heavier than air and spread along ground. Hydrogen UN1049 Deuterium Hydrogen refrigerated liquid UN1966 and Methane are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame.
Use an alternate method of detection thermal camera broom handle. 14 Hydrogen fluoride is an excellent solvent. Experts are waiting 247 to provide step-by-step solutions in as fast as 30 minutes.
In this approach HF is oxidized in the presence of a hydrocarbon and the fluorine replaces CH bonds with CF bonds. There are different methods for the preparation of hydrogen fluoride gas. Synthesis structure and reactions.
HF reacts with Lewis. 14 HF is the precursor to elemental fluorine F2 by electrolysis of a solution of HF and potassium bifluoride. 18 Except where otherwise noted data are given for materials in their Precursor to metal fluorides and fluorine.
Hydrogen fluoride is a colorless gas that is corrosive in nature. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is. 70 More Lewis Dot Structures.
This is due to hydrogen bonding that occurs between molecules. Unlike the other hydrogen halides HF is lighter than air and it is particularly penetrating which can damage the lungs. Aqueous solutions of HF called.
Lewis Structure electron dot diagram for hydrogen fluoride OR. The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom which may or may not be circled are referred to as a covalent bond or a single covalent bond. Name _____ Lewis Dot Structures of Ionic Compounds Date_____ Chemistry.
Carbonyl fluoride COF2 or CF2O CID 9623 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. A strong acid p Ka -10. Not to be confused with hydroiodic acid which is an aqueous solution of hydrogen iodide and best described as H 3 O I -.
Molecular Structure of Hydrogen Iodide. Lewis hydrogen fluoridegif 120 60. 1 KB Lewisschreibweise mit Verdeutlichung der Wasserstoffbrückensvg 965 398.
55 KB Molecular orbital of hydrogen fluoridesvg 241 190.