
Gold cannot be produced via chemistry or alchemy. Red cinnabar α-HgS is the form in which mercury is most commonly found in nature.
Isotopes are two or more forms of an element.
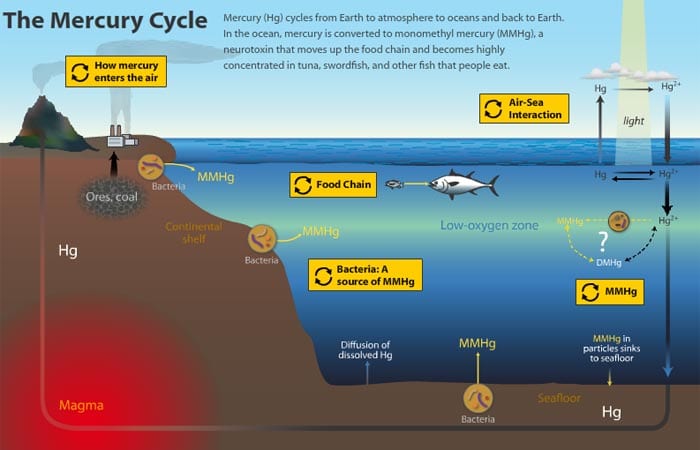
Forms of mercury in nature. Natural sources of atmospheric mercury include volcanoes geologic deposits of mercury and volatilization from the ocean. Although all rocks sediments water and soils naturally contain small but varying amounts of mercury scientists have found some local mineral occurrences and thermal springs that are naturally high in mercury. In nature especially in certain types of wetlands deposited mercury can chemically change into a toxic form called methylmercury.
Toxic air pollutants such as pesticides PCBs flame retardants PBDEs and other industrial or combustion by-products are generally manmade compounds. Mercury has three forms. Elemental liquid mercury inorganic mercury and organic mercury methylmercury.
Elemental mercury is the most common form. It is a metallic silvery liquid also referred to as quicksilver that is processed from an ore called cinnabar. Mercury Mercury is an element and a metal that is found in air water and soil.
It exists in three forms that have different properties usage and toxicity. The three forms are called elemental or metallic mercury inorganic mercury compounds and organic mercury compounds. Elemental mercury is liquid at room temperature.
The largest natural source of mercury is cinnabar its only known ore and the richest deposits are found in Spain and Italy. This reddish mineral containing mercury. Mercury occurs uncombined in nature to a limited extent.
The metal is obtained commercially from cinnabar a mercuric sulfide ore. It is easily separated by roasting the ore in air. The metal is usually purified by repeated vacuum distillation.
Mercury metal has many uses. HgS is dimorphic with two crystal forms. Red cinnabar α-HgS is the form in which mercury is most commonly found in nature.
Black metacinnabar β-HgS is less common in nature and adopts the wurtzite crystal structure. 11 Mercury is a heavy metal sometimes known as quicksilver that occurs naturally in the environment in different chemical forms. The pure form elemental mercury is liquid at room temperature and slowly forms a vapour in the air.
Forms more commonly found in. Mercury Compounds Hazard Summary Mercury exists in three forms. Elemental mercury inorganic mercury compounds primarily mercuric chloride and organic mercury compounds primarily methyl mercury.
All forms of mercury are quite toxic and each form exhibits different health effects. There are three main types of mercury such as elemental mercury Organic mercury and Inorganic mercury. Elemental mercury is a liquid at the room temperature.
Seven naturally occurring isotopes of mercury are known. They are mercury-196 mercury-198 mercury-199 mercury-200 mercury-201 mercury-202 and mercury-204. Isotopes are two or more forms of an element.
Isotopes differ from each other according to their mass number. Natural sources of mercury Mercury is an element that occurs naturally throughout our solar system. On Earth geological deposits are most often found in the form of a mercury sulfide mineral.
The mercury content in this mineral the most important ore of mercury can reach 86. When mercury enters the water it can combine with organic materials to form organic compounds such as methylmercury MeHg which is produced mainly by bacteria and is the form that poses the greatest risk to environmental exposure. The process by which mercury contaminates fish is very complex.
Simply put tiny organisms absorb mercury. Mercury exists in three formselemental mercury pure metallic mercury organic mercury mainly methylmercury the form found in some fish and inorganic mercury such as the mercury ll sulfide that makes up cinnabar. Unlike organic mercury inorganic mercury doesnt contain carbon.
Theoretically its possible to form gold by the nuclear processes of fusion fission and radioactive decay. Its easiest for scientists to transmute gold by bombarding the heavier element mercury and producing gold via decay. Gold cannot be produced via chemistry or alchemy.
Chemical reactions cannot change the number of protons within an atom.