MCB Manufacturing Chemists Norwood Ohio. 4 NMR 4 FTIR 2 UV-Vis and 2 MS.
3 -Phenylpropanal 3 PPM Proton NMR integral values 40 PPM Carbon 13 NMR.
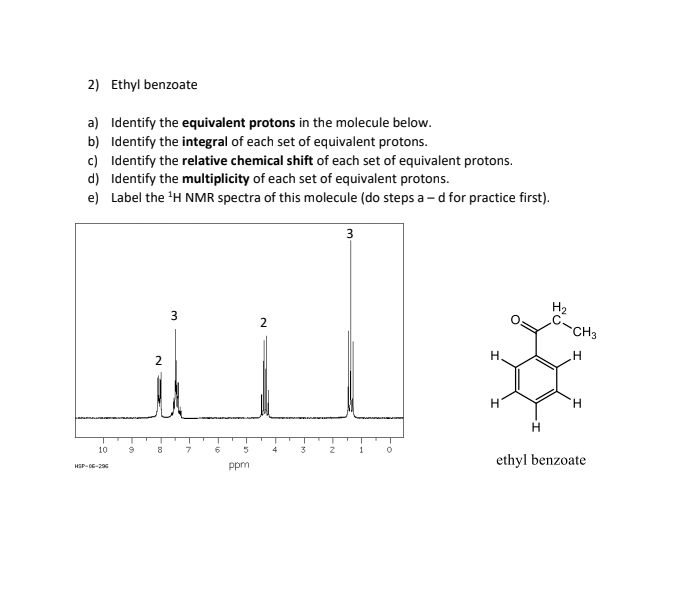
Ethyl benzoate proton nmr. ChemicalBook ProvideEthyl benzoate93-89-0 1H NMRIR2MSIR3IR11H NMRRamanESR13C NMRSpectrum. Ethyl benzoate C9H10O2 CID 7165 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. 1D NMR Spectrum 2707 - Ethyl benzoate HMDB0033967 1D NMR Spectrum 3399 - Ethyl benzoate HMDB0033967 Human Metabolome.
NMR Kovats Ions Semiochemicals Taxa Synthesis Control Invasive spp. Previous Compound ethyl alcohol Next Compound ethyl butyrate NMR - Compound ethyl benzoate. 15017 Behavioural function Kovats Synthesis Dots surface.
Proton NMR 8 integral values Carbon 13 NMR. 3 -Phenylpropanal 3 PPM Proton NMR integral values 40 PPM Carbon 13 NMR. Ethyl benzoate integral values Carbon 13 NMR 2 PPM 20 PPM.
Propanolc acid PPM integral values Proton NMR 20 Carbon 13 NMR. Propyl acetate 45 Proton NMR Carbon 13 NMR. Home A Level Proton NMR Introduction Ethyl Benzene Proton NMR Equivalent Protons.
Ethyl Benzene Proton NMR Equivalent Protons. 0 0 Click the 2D protons and the coloured spectrum peaks to view the respective 3D models. Protons in chemically equivalent environments appear in the same place in the spectrum.
H And 13C NMR Spectra Of Ethyl Benzoate Acquired In CDC13 TMS Internal Standard With A Bruker Avance 300 MHz Instrument Are Shown In Your Manual with Expansions In Some Regions. Refer To These Spectra Data And The Notes In The Manual To Complete The Following Table To Help Assign The Data ii Formally Record The H NMR Data In The Style Described. All hydrogen nuclei protons are visible in NMR any protons in the solvent molecules will likely give off a much stronger signal than protons in the sample since there are so many more molecule of solvent.
Ethyl Benzoate folder. Once you open the file the. Proton Spectrum of Ethylbenzene with SpinSpin Coupling.
The description of proton NMR spectra thus far has been greatly simplified by the fact that all signals with the exception of those from the benzene ring in benzylacetate have been singlets. The structure of the organic compound ethylbenzene and the corresponding proton spectrum are illustrated in the Ethylbenzene figure and the Ethylbenzene. 13C Nuclear Magnetic Resonance NMR Chemical Shifts.
View the Full Spectrum for FREE. View the Full Spectrum for FREE. The full spectrum can only be viewed using a FREE account.
BENZOIC ACID ETHYL ESTER. MCB Manufacturing Chemists Norwood Ohio. Compound P-Dimethylamino-benzoic acid ethyl esterwith free spectra.
4 NMR 4 FTIR 2 UV-Vis and 2 MS. The triplet for the methyl peak means that there are two neighbors on the next carbon 3 - 1 2H. The quartet for the methylene peak indicates that there are three hydrogens on the next carbon 4 - 1 3H.
Table NMR 1 summarizes coupling patterns that arise when protons. Ethyl 2-methylbenzoate C10H12O2 CID 66598 - structure chemical names physical and chemical properties classification patents literature biological. Methyl benzoate 1H NMR 60 MHz 1 scan 11 seconds 1H NMR spectrum of methyl benzoate shows two multiplets in the aromatic region and one singlet upfield.
Integration of the signals and understanding the deshielding effect of the methyl ester group helps further interpretation. Methyl benzoate 13C NMR 15 MHz 30 pulse 64 scans 8 minutes. Integration of 13 C NMR Spectra.
In a 1 H NMR spectrum the area under the signals is proportional to the number of hydrogens giving rise to the signal. As a result the integration of the spectrum is a measure of the proton count. In a 13 C NMR spectrum the area under the signal is not simply proportional to the number of carbons giving rise to the signal because the NOE from proton decoupling.
Assign as many resonances as you can to specific carbon atoms in the 13 C NMR spectrum of ethyl benzoate. It is very obvious on NMR that C is the benzoate since the 27 2H is very typical for protons on the carbon next to the carbonyl. D is the propanoate since the 44 2H is for protons.
These results confirm that the alkoxyphosphonium benzoate is an intermediate in ethyl benzoate formation and that it is possible for this salt to be identified by proton NMR. In fact each of the main species involved in alkyl benzoate synthesis via the Mitsunobu reaction can be detected by proton NMR. 6 protons This page requires the MDL Chemscape Chime Plugin.
