NH 4 acetate ion. The compound structure is made by 1 hydroxide anion OH and 1 ammonium cation NH 4 and these share an ionic bond.
Mentionable that bi-phosphate HP4 2- is a bivalent ion wherease ammonium NH4 is.
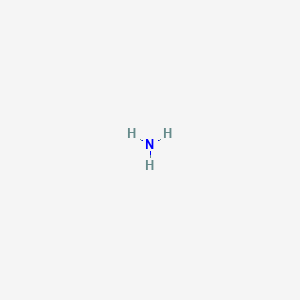
Chemical formula of ammonium ion. 7 righe Ammonium ion hydridonitrogen1 UNII-54S68520I4. The ammonium ion is a positively charged ion or cation and has the chemical formula NH 4. Ammonium salts are commonly used in fertilizers.
Examples of compounds containing the ammonium ion include ammonium nitrate NH 4 NO 3 and ammonium sulfate NH 4 2 SO 4. All ammonium salts are soluble in water and dissociate or break down to produce. Molecular Formula H 4 N.
Average mass 18038 Da. Monoisotopic mass 18033827 Da. Formula Properties and Uses.
He Ion ammonia Is a positively charged polyatomic cation whose chemical formula is NH 4. The molecule is not flat but has the shape of a tetrahedron. The positively charged ammonium ion NH 4 The four hydrogen atoms make up the four corners of the tetrahedron.
Ion Name Ion Formula. NH 4 hydroxide ion. CO 3 2.
Bicarbonate or hydrogen carbonate. C 2 H 3 O 2 or CH 3 CO 2. SO 4 2.
SO 3 2. PO 4 3. PO 3 3.
NH 4 acetate ion. C 2 H 3 O 2 also written CH 3 CO 2 carbonate ion. Cr 2 O 7 2.
Hydrogen carbonate ion bicarbonate ion HCO 3. Ammonium is a cation having the chemical formula NH 4. Ammonium ion is an inorganic ion composed of one nitrogen atom and four hydrogen atoms.
Methanamine conjugate acid 9CI. In this video well write the correct formula for Ammonium phosphate NH43PO4To write the formula for Ammonium phosphate well use the Periodic Table a. Answered 2 years ago Author has 55 answers and 606K answer views The formula is NH42 HPO4.
Mentionable that bi-phosphate HP4 2- is a bivalent ion wherease ammonium NH4 is. The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. It is formed by the protonation of ammonia NH 3.
Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR. Ammonium Chloride Chemical Formula NH 4 Cl. Ammonium chloride is used in several sectors like in fertilizers glue medicines food additives cooling baths and as an acidifier.
There are various other uses of NH4Cl but its use should be limited due to the environmental hazards it poses. The chemical formula of the ammonium hydroxide is NH4OH. The molar mass is 3504 gmol -1.
The compound structure is made by 1 hydroxide anion OH and 1 ammonium cation NH 4 and these share an ionic bond. An image showing its chemical structure is below in the common representations that we generally use for the organic molecules. Ammonium is an ionized form of ammonia.
The chemical structure for ammonium is NH 4. The chemical structure for ammonia is NH 3. The chemical formula of this compound is NH 4 NO 3.
Ammonium nitrate readily dissolves in water by dissociating into its constituent ions. This salt is acidic in nature since it is derived from a weak base NH 3 and a strong acid HNO 3. SYNONYMS Ammonium bicarbonate INS No.
503ii DEFINITION Chemical names Ammonium hydrogen carbonate CAS. Number 1066-33-7 Chemical formula CH 5NO 3 Structural formula NH 4HCO 3 Formula weight 7906 Assay Not less than 990 DESCRIPTION White crystals or a crystalline powder with a slight odour of ammonia. FUNCTIONAL USES Raising agent CHARACTERISTICS.
