AD is a slowly progressive disorder with insidious onset and progressive impairment of episodic memory and executive function coupled with aphasia apraxia and agnosia 1. Rat and mouse amyloid-beta peptides bind only weakly transient metals and have little reducing activity due to substitutions of transient metal chelating residues.
In Alzheimers disease AD Aβ self-assembles into metastable toxic oligomers that injure neurons and synapses disrupt neuronal communication and cause gradual neurodegeneration in susceptible brain areas predominantly those responsible for memory and cognitive function.
Amyloid beta protein structure. 6 rânduri Amyloid beta-protein 17-42 C119H194N28O33S CID 16131051 - structure chemical names. C Solution structure of amyloid beta peptide 140 in which the C-terminal two-thirds of the peptide form an alpha-helix conformation between residues 15 and 36 with a. Structure biology and structure-based therapeutic development.
Amyloid beta peptide Aβ is produced through the proteolytic processing of a transmembrane protein amyloid precursor protein APP by β- and γ-secretases. Aβ accumulation in the brain is proposed to be an early toxic event in the pathogenesis of Alzheimers disease. Amyloid beta-Protein 22-35 C59H102N16O21S CID 3605362 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
The amyloid β-protein Aβ is a seminal neuropathic agent in Alzheimers disease AD. Recent evidence points to soluble Aβ oligomers as the probable neurotoxic species. The 3D structure of the Aβ 142 protofilament consists of two stacked intermolecular parallel in-register β-sheets that perpetuate along the fibril axis Fig.
4 A and B. The residues L17 F19 and A21 of sheet β1 mediate the hydrophobic intermolecular contacts with. Total Structure Weight.
The aggregation of neurotoxic amyloid-β Aβ polypeptides into aberrant extracellular senile plaques is the major neuropathological hallmark of Alzheimers disease AD. In this review we analyze issues concerning processes of generation of two proteins β-amyloid peptide and Tau-protein in the cell which are believed to play the key role in AD genesis. Until recently these agents were considered independently of each other but in light of the latest studies it becomes clear that it is necessary to study their interaction and combined effects.
The major components of neuritic plaques found in Alzheimer disease AD are peptides known as amyloid beta-peptides Abeta which derive from the proteolitic cleavage of the amyloid precursor proteins. In vitro Abeta may undergo a conformational transition from a soluble form to aggregated fibrillary beta-sheet structures. AD is a slowly progressive disorder with insidious onset and progressive impairment of episodic memory and executive function coupled with aphasia apraxia and agnosia 1.
The amyloid β-protein Aβ appears to play an essential role in the pathogenesis of AD. Amyloid-beta peptides are lipophilic metal chelators with metal-reducing activity. Binds transient metals such as copper zinc and iron.
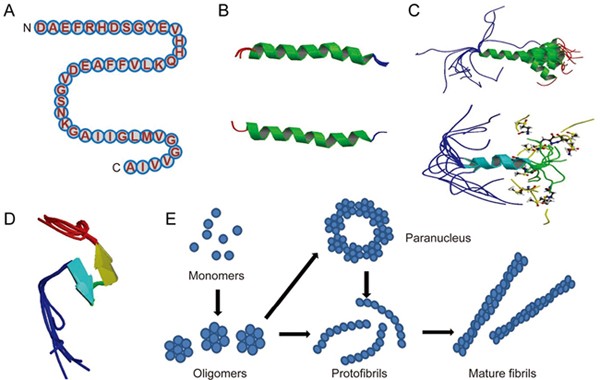
Rat and mouse amyloid-beta peptides bind only weakly transient metals and have little reducing activity due to substitutions of transient metal chelating residues. Looking in the PDB you can find structures of the amyloid-beta peptide in its two guises. The structure on the left PDB entry 1iyt shows the peptide as it might look when interacting with the membrane.
Most of it is folded into an alpha helix forming a more-or-less extended structure. Amyloid β-protein Aβ is a small natively unstructured protein with unknown physiological function. In Alzheimers disease AD Aβ self-assembles into metastable toxic oligomers that injure neurons and synapses disrupt neuronal communication and cause gradual neurodegeneration in susceptible brain areas predominantly those responsible for memory and cognitive function.